About Customers
As one of the earliest multinational pharmaceutical enterprises and the largest pharmaceutical subsidiary of Johnson&Johnson in China, Xi’an Janssen Pharmaceutical Ltd. was founded in 1985 with its headquarters in Beijing and its production base in Xi'an, with more than 3000 employees in China. The company's business includes the production and sales of high-quality drugs, and its products mainly involve gastroenterology, neuropsychiatry, allergology, pain management, anti-infection, biological agents and tumor and other fields.

Project background
GS1 is a coding standard widely adopted by the global pharmaceutical industry, but not recognized and adopted by the National Medical Products Administration of China before 2019. In the past, imported drugs need to be unpacked and re-coded and labeled in the bonded warehouse when they are transported from foreign places of origin to China. It is not only time-consuming and labor-intensive, but also costly. More importantly, the traceability data chain of the supply chain is broken, which makes it impossible for pharmaceutical enterprises to build a global digital supply chain with end-to-end industry chain traceability and efficient collaboration based on unified code assignment rules and standards, nor can they better connect patients and maintain patient medication safety.
In 2019, the NMPA issued a new version of the traceability code specification to achieve international integration at the code assignment standard level. In this context, Acctrue Technology has cooperated with Xi'an Janssen and Tencent Intelligent Medicine to build this platform project. The platform innovatively applied GS1 to solve the problems of global regulatory compliance, serialized data docking, whole-industry chain traceability and digital operation of imported pharmaceutical products in a one-stop way, helping Xi'an Janssen to realize the construction of end-to-end traceability system for the pharmaceutical industry, enabling the efficient coordination of all elements of the pharmaceutical supply chain and the efficiency improvement of the whole industrial chain. So far, the project has opened up more than 160 circulation enterprises and more than 400 hospitals, and is connected with the medical insurance system and the patient terminal.
Customer demands
The traceability and compliance of the whole process of the circulation and sales of imported drugs from the foreign country of origin to the territory of the applicant country based on the GS1 code assignment standard has been realized, providing the end-to-end traceability information of the whole process;
Clearly understand the inventory, flow direction and flow rate of products in all links of the supply chain;
With the help of Tencent's capabilities on the client side, the packaged pharmaceutical enterprises reach patients and provide patient medication services.
Solutions
According to customers' demands, Acctrue Technology has provided Xi'an Janssen customers with a set of solutions for the construction of a digital platform for the traceability of the whole industrial chain of imported drugs GS1 based on the "Acctrue Traceability Cloud (linklink)" platform. At the same time, it has cooperated with Tencent to solve the problems of global regulatory regulation, serialized data docking, traceability of the whole industrial chain and digital operation of imported drugs based on the GS1 international standard through docking with the "Tencent Health Medicine Box" small program, It helped Xi'an Janssen realize the end-to-end traceability system construction of the pharmaceutical industry, enabling the efficient collaboration of all elements of the pharmaceutical supply chain and the efficiency improvement of the entire industrial chain. At present, the project has opened more than 160 circulation enterprises and more than 400 hospitals, and is connected with the medical insurance system and the patient terminal.
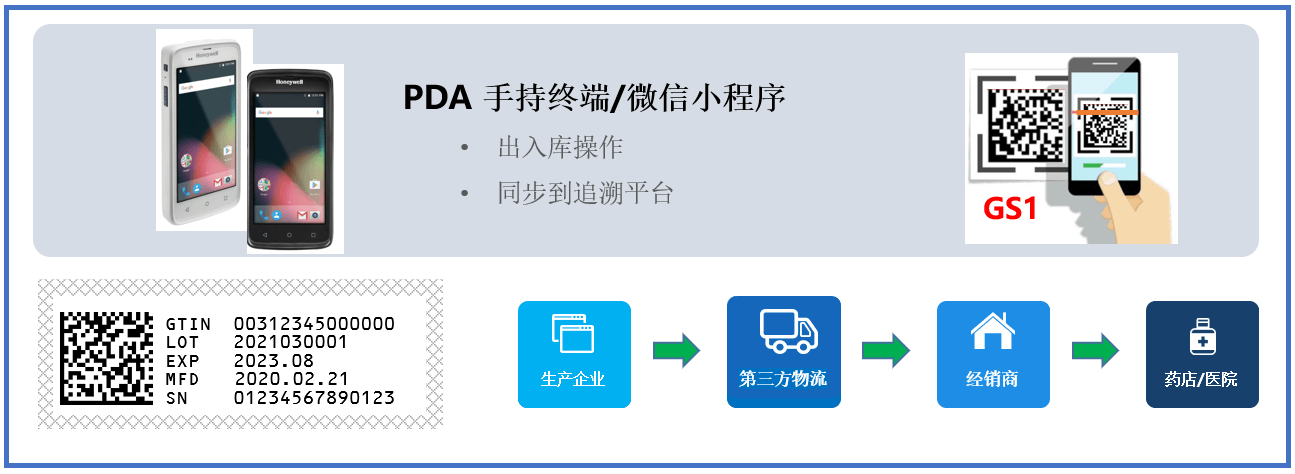
First, connecting with GS1 code assignment of the country of origin
The GS1 DataMatrix is assigned to each sales unit in the production line of the country of origin according to the international common standard. The data includes five international common elements: GTIN code, unique serial number, production date, expiration date and product batch number. The GS1 international code assignment standard is applied to complete the assignment of packaging materials at all levels, and the JB is associated;
Second, interfacing with production and circulation data
Connect the production data through SAP-ICH and the Janssen WMS system at the same time to collect and synchronize the in-and-out data of drugs in the domestic bonded warehouse (Actelion) and third-party logistics, so as to minimize the impact on the existing process;
Third, terminal data uploading
Retail pharmacies use Janssen's "Senxin Care" system to complete the receipt. The "Senxin Care" system obtains the delivery information of the upstream dealers through the interface developed by Acctrue to ensure that the data is delivered to the "Senxin Care" system in a timely, complete and accurate manner.
Forth, connecting with national supervision/coordination platform and medical insurance system
Connect the national drug traceability supervision platform, drug traceability collaborative service platform and medical insurance authentication system through Acctrue traceability cloud platform.
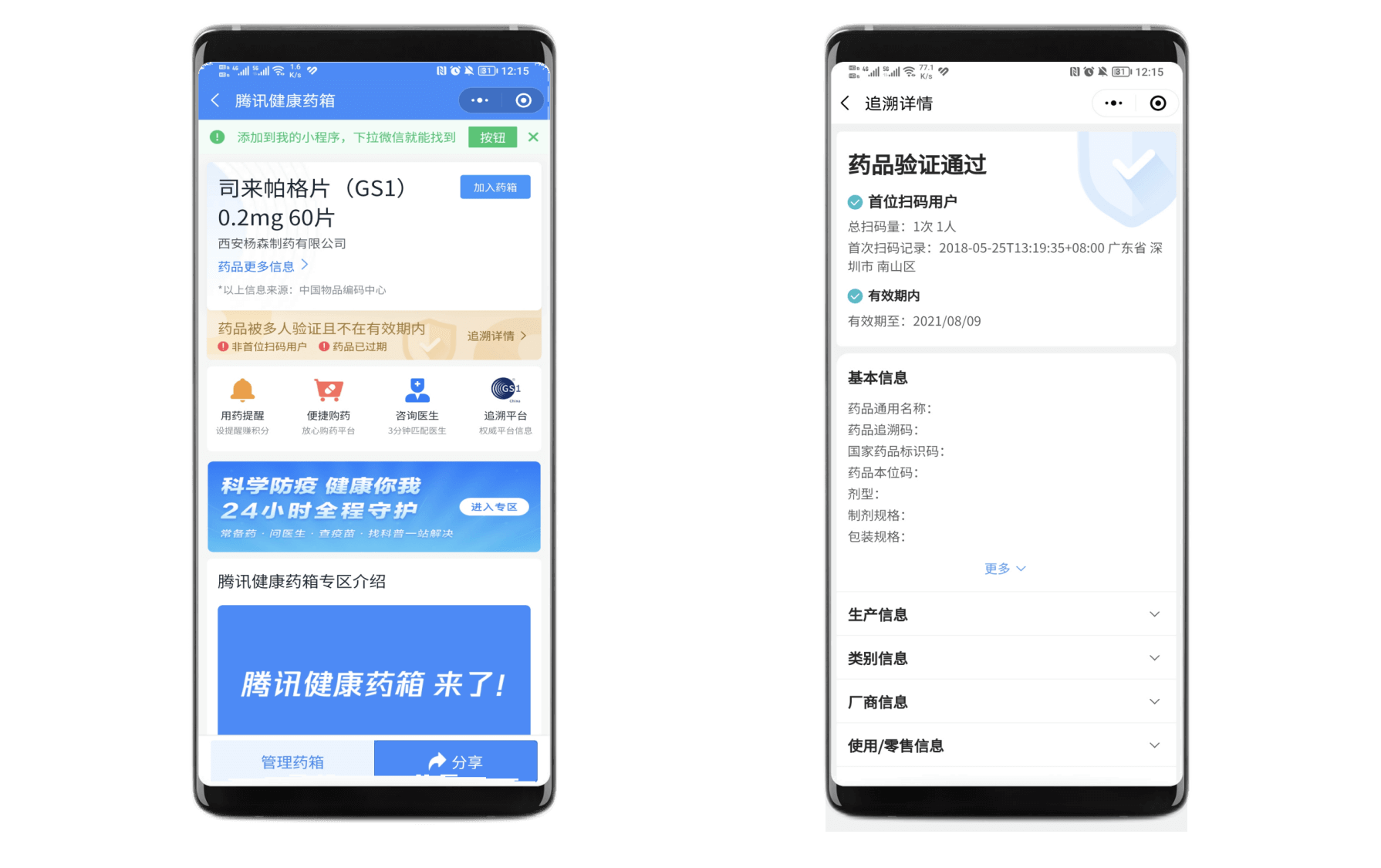
Fifth, connecting with Tencent WeChat widget "Tencent Health Medicine Box (Intelligent Medicine)”
Patients can easily identify the authenticity, query the traceability information and obtain a variety of intelligent pharmaceutical services by scanning the traceability code of the drug packaging box through WeChat.
Project achievements
1 Build a data center for traceability of imported drugs
Based on the metadata management of drug serialization and whole-production chain traceability, the center of drug traceability data was established, which laid a data foundation for the efficiency and empowerment of the supply chain;
2 Supply chain digital collaboration
A full-process identification of inbound and outbound scanning codes is established through the collection and sharing of data from pharmaceutical enterprises, third-party logistics, dealers, and pharmaceutical companies, enabling the efficient collaboration of all elements of the pharmaceutical supply chain, and realizing the construction of the end-to-end traceability system of the pharmaceutical industry;
3 Applying GS1 to improve the efficiency of the whole industrial chain
Through the construction of the platform, pharmaceutical enterprises, operating companies, medical institutions and patients are connected end-to-end to realize the data collaboration and sharing of the whole supply chain, improve the efficiency of the supply chain and the role of patient safety, bring about the industry demonstration effect, and provide model cases for imported pharmaceutical enterprises to connect with China's third-party traceability platform.



































